|
 |
![]()
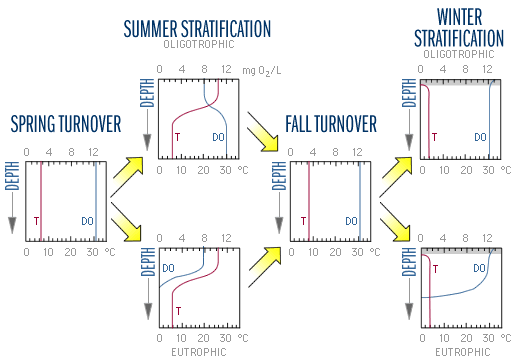
Biological activity peaks during the spring and summer when photosynthetic activity is driven by high solar radiation. Furthermore, during the summer most lakes in temperate climates are stratified. The combination of thermal stratification and biological activity causes characteristic patterns in water chemistry. Figure 9 shows the typical seasonal changes in dissolved oxygen (DO) and temperature. The top scale in each graph is oxygen levels in mg O2/L. The bottom scale is temperature in °C. In the spring and fall, both oligotrophic and eutrophic lakes tend to have uniform, well-mixed conditions throughout the water column. During summer stratification, the conditions in each layer diverge.
The DO concentration in the epilimnion remains high throughout the summer because of photosynthesis and diffusion from the atmosphere. However, conditions in the hypolimnion vary with trophic status. In eutrophic (more productive) lakes, hypolimnetic DO declines during the summer because it is cut-off from all sources of oxygen, while organisms continue to respire and consume oxygen. The bottom layer of the lake and even the entire hypolimnion may eventually become anoxic, that is, totally devoid of oxygen. In oligotrophic lakes, low algal biomass allows deeper light penetration and less decomposition. Algae are able to grow relatively deeper in the water column and less oxygen is consumed by decomposition. The DO concentrations may therefore increase with depth below the thermocline where colder water is "carrying" higher DO leftover from spring mixing (recall that oxygen is more soluble in colder water). In extremely deep, unproductive lakes such as Crater Lake, OR, Lake Tahoe, CA/NV, and Lake Superior, DO may persist at high concentrations, near 100% saturation, throughout the water column all year. These differences between eutrophic and oligotrophic lakes tend to disappear with fall turnover (Figure 9).
In the winter, oligotrophic lakes generally have uniform conditions. Ice-covered eutrophic lakes, however, may develop a winter stratification of dissolved oxygen. If there is little or no snow cover to block sunlight, phytoplankton and some macrophytes may continue to photosynthesize, resulting in a small increase in DO just below the ice. But as microorganisms continue to decompose material in the lower water column and in the sediments, they consume oxygen, and the DO is depleted. No oxygen input from the air occurs because of the ice cover, and, if snow covers the ice, it becomes too dark for photosynthesis. This condition can cause high fish mortality during the winter, known as "winter kill." Low DO in the water overlying the sediments can exacerbate water quality deterioration, because when the DO level drops below 1 mg O2/L chemical processes at the sediment-water interface frequently cause release of phosphorus from the sediments into the water. When a lake mixes in the spring, this new phosphorus and ammonium that has built up in the bottom water fuels increased algal growth.