 |
 |
Physical and Chemical Processes
The physical characteristics and chemistry of water change as water comes in contact with air, soil, rocks, bacteria, vegetation and biological communities. This section provides a brief description of the physical materials and chemical processes influencing stream water quality. As water moves along pathways in a watershed, eroded soil and plant materials enter the flowing water. Addition of these materials, in conjunction with chemical and biological processes operating within the river, influences the physical and chemical properties of the flowing water (Figure 1.7). External (or allochthonous) materials entering the river include eroded soils, salts carried by rain or leached from rock and soil, and leaves and woody material washed or dropped into the stream. Internal (or autochthonous) processes operating within the river include physical breakdown of rocks and plant materials, microbial decomposition of organic matter, cycling of carbon and dissolved nutrients in the presence of sunlight by plants and animals, and chemical transformations of inorganic ions under changing conditions of temperature, pH, and oxygen concentration.
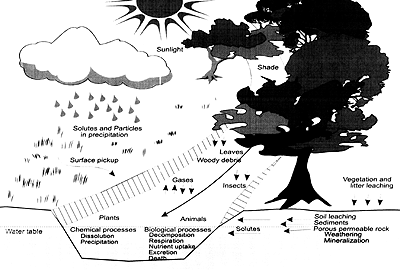
Figure 1.7 Materials and Processes Contributing to the Physical and Chemical Properties of a Stream
The chemical constituents resulting from these external and internal processes may be suspended or dissolved in the stream water. Suspended particles are classified either as sediment or colloids, depending upon their size. Suspended particles measuring 0.1 mm or greater in diameter are considered coarse sediment and they will settle rapidly out of still water. Smaller suspended particles include silts, clays, and organic particles (sometimes called organic detritus) derived from plants and animal activity. Bacteria and suspended algae (phytoplankton) also are in this size range (see next section). Clay-sized particles (< 63 m) settle very slowly, even in still water, unless they become coagulated into larger particles. Colloidal particles are smaller than about 0.5 m in diameter. Although colloids are too small to be seen with the naked eye, they provide surfaces for absorption of dissolved chemicals and affect water color and clarity (turbidity).
Dissolved constituents include organic compounds, gases, and inorganic ions (salts) such as calcium, magnesium, and sodium. Some inorganic chemicals, such as phosphorus, are common in all natural stream waters and are beneficial and even necessary for life. For example, phosphorus is an essential nutrient for plant life. These solutes are considered pollutants only when their levels are elevated to the point where they threaten the health of the ecosystem. Elevated concentrations of trace constituents are usually linked to anthropogenic factors, such as industrial discharges or runoff carrying agricultural fertilizers.
Stream water chemistry can vary both daily and seasonally. Much of this variability results from changes in the proportions of stormflow and baseflow, which often have very different chemical properties. Water chemistry also is affected by changes in the amount of flow. While periods of high flows decrease the concentration of some point source pollutants through dilution, they also may increase nonpoint source pollutants, such as those from atmospheric deposition, that accompany higher runoff.